Introduction: What is UV-Visible Spectroscopy?
 |
 |
 |
 |
How do we precisely measure the color of a solution, or the purity of a DNA sample? How can we quantify the concentration of a specific compound in a complex mixture? The answer lies in a powerful and versatile analytical technique: UV-Visible (UV-Vis) spectroscopy.
In simple terms, UV-Vis spectroscopy is a method used to measure how much a chemical substance absorbs light in the ultraviolet (UV) and visible portions of the spectrum. By analyzing the interaction between light and matter, this technique allows for both the qualitative identification and quantitative analysis of a vast range of chemical and biological compounds. It is a fundamental tool used in virtually every modern scientific laboratory, from academic research to industrial quality control.
The foundation of spectrophotometry is that every chemical compound absorbs, transmits, or reflects light over a specific range of wavelengths. The absorption of light is due to the interaction of photons with the electronic and vibrational modes of the molecules. Because each type of molecule has a unique set of energy levels, it will only absorb light of specific wavelengths, resulting in a unique spectral “fingerprint”.
This relationship is quantified by the Beer-Lambert Law, which states that there is a linear relationship between the absorbance of a solution and the concentration of the analyte within it. The law is expressed by the simple formula:
A=−log10ItI0=log101T=K⋅l⋅c
Where:
- A is Absorbance (a unitless measure).
- K is the molar absorptivity, a constant unique to each substance at a given wavelength.
- l is the path length, or the distance the light travels through the sample (typically the width of the cuvette, e.g., 1 cm).
- c is the concentration of the substance in the solution(g/L or mol/L).
Essentially, a more concentrated solution or a longer path length will result in more light being absorbed. If the light path remains constant, higher absorbance indicates greater solution concentration. This intuitive principle forms the basis for precise quantitative analysis.
The Core Instrument: A Brief Introduction to the Spectrophotometer
The spectrophotometer is the precision instrument that brings the principles of UV-Vis spectroscopy to life. It is designed to measure the amount of light (photons) absorbed after it passes through a sample solution.
A typical spectrophotometer consists of four core components working in seque:
 |
 |
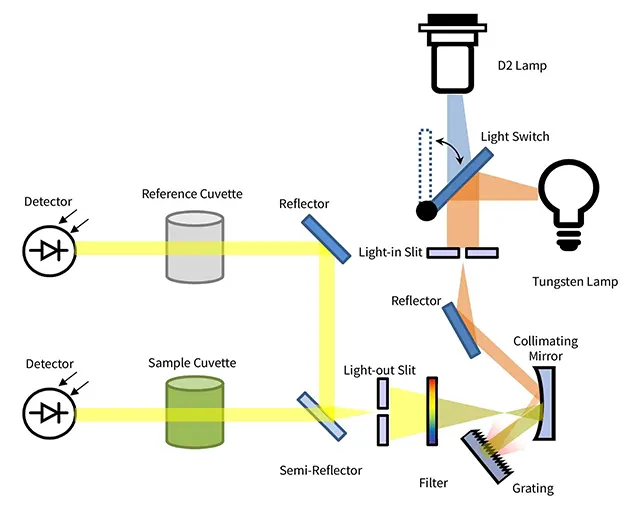 |
- Light Source: A lamp (often a combination of Deuterium for UV and Tungsten for visible light) provides a stable, continuous spectrum of light.
- Monochromator: This component, typically a diffraction grating, acts like a prism. It separates the white light from the source into its constituent wavelengths, allowing only a very narrow, specific wavelength to be selected and passed toward the sample.
- Sample Compartment: This holds the sample, usually contained within a small, transparent vessel called a cuvette, in the path of the selected light beam.
- Detector: After passing through the sample, the remaining light strikes a detector (such as a photomultiplier tube or photodiode). The detector measures the intensity of the transmitted light and converts it into a digital signal, which is then displayed as an absorbance or transmittance value.
Technical Guides: Understanding Core Principles and Technological Differences
From fundamental theory to instrument comparisons, this series of articles builds a solid foundation of technical knowledge.
- An in-depth look at the Beer-Lambert Law and the relationship between absorbance and transmittance.
- Which instrument is right for your application?
- How does a spectrophotometer work?
Application Notes: Exploring the Endless Possibilities of the Spectrophotometer
Calibration & Operations: Best Practices for Accurate and Reliable Measurements
Buyer’s Guides: Make a Smart Investment by Choosing the Right Spectrophotometer
From budget planning to brand comparisons, we help you make an informed purchasing decision.
Conclusion
UV-Visible spectroscopy is a powerful, versatile, and indispensable technique in the modern laboratory. Its high sensitivity, precision, and speed make it essential for everything from fundamental biochemical research and drug development to ensuring the quality of our food and the safety of our environment. By understanding its core principles and wide-ranging applications, you can unlock its full potential to drive innovation and ensure quality in your work.
